What is Mass Number?
In this article, we will learn how to calculate mass number(A) but first off, lets define it. Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus.
Neutrons are the subatomic particles present in atomic nuclei like protons. These have no net charge and have mass slightly more than that of protons. These are the constituent of the nucleus of all atoms except for hydrogen.
While electrons are the subatomic particles like protons and neutrons but located outside the nucleus and are negatively charged. These are the primary carriers of electricity. They have negligible mass
The mass number allows us to tell the difference between elements with a different number of neutrons, called isotopes.
Characteristics of A.
- The mass number is written as a superscript on the left side of the symbol of the element.
- The mass number for all the elements is always a whole number. It cannot be a fraction.
- The mass number is unit less.
Formula to Calculate Atomic Mass Number.
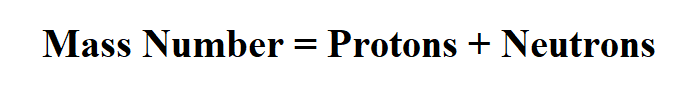
Example 1:
The hydrogen atom doesn’t have a neutron but it has 1 proton, so the mass number of hydrogen is 1.
Example 2:
Oxygen atom has 8 protons and 8 neutrons. Determine the mass number.
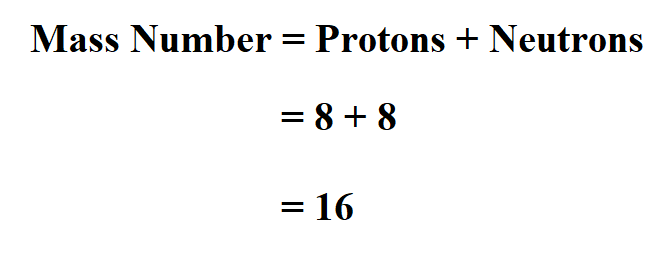
Thus, oxygen atom mass number is 16.