Osmosis is the movement of water from an area of low concentration of solute to an area of higher concentration of solute.
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane or simply the pressure that stops the process of osmosis.
Formula to calculate osmotic pressure.
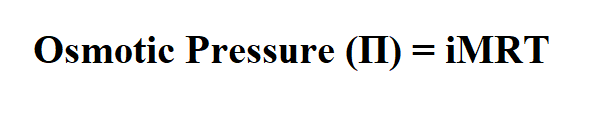
M is the molar concentration of dissolved species. i is the van ‘t Hoff factor of the solute.
R is the ideal gas constant (0.08206)
T is the temperature on the Kelvin scale.
Example:
Calculate the osmotic pressure of 0.15 mol/L sucrose with the van’t Hoff factor as 1 if its absolute temperature is 298K.
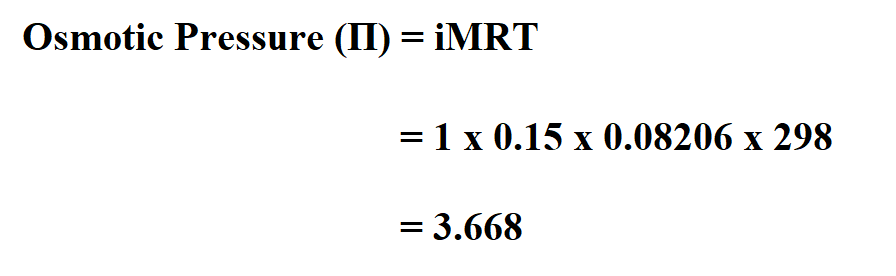
Therefore, it’ osmotic pressure is 3.668 atm.

