In this article we will define and discuss how to calculate buffer capacity but before that, lets define a buffer. Buffers are compounds that resist changes in pH upon the addition of limited amounts of acids or bases.
Additionally, there are two type of buffer capacity and they are;
- Acidic buffer. It is formed by mixing a solution of a weak acid and the salt of this weak acid with a strong base.
- Basic buffer. It is formed by mixing a solution of a weak base and the salt of this weak base with a strong acid.
Buffer capacity is a quantitative measure of resistance to pH change upon the addition of H+ or OH- ions. It depends on the amount of acid and base used to prepare the buffer.
Further, if the buffer capacity is 10 times larger, then the buffer solution can absorb 10 times more strong acid or base before undergoing a significant change in pH.
Formula to Calculate Buffer Capacity.
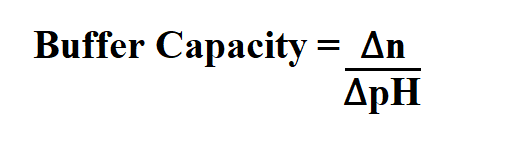
∆n is the equivalents of strong base or acid added per volume liter.
∆pH is the change in pH.
Example:
Find the buffer capacity if you add 0.080 mole of HCl to 100ml phosphate buffer and the pH drops exactly one unit .
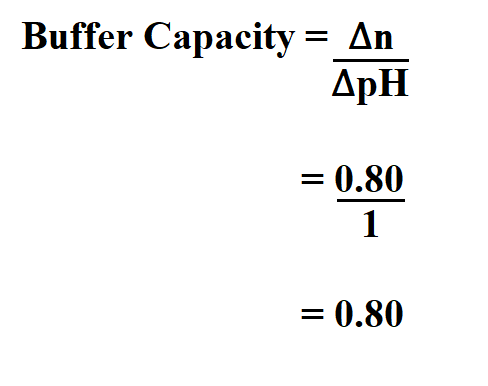
Therefore, the buffer capacity is 0.80.