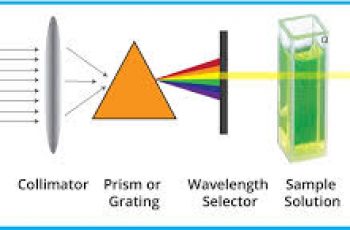
Vapor pressure is a measure of the tendency of a material to change into the gaseous or vapor state, and it increases with temperature.
Vapor pressure is constant when there is an equilibrium of water molecules moving between the liquid phase and the gaseous phase, in a closed container.
Formula to calculate vapor pressure.

Example:
Suppose the vapor pressure of water is 25.756 mm Hg at 25 °C, if the mole fraction of the solvent is 0.92405, calculate the vapor pressure of the solution.

Therefore, the vapor pressure is 23.80mm Hg.