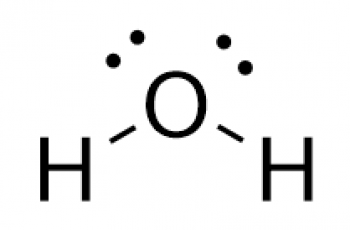
Formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula.
It is expressed in atomic mass units.
Formula mass (M) is used for a substance made up of molecules.
Formula to calculate formula mass.

Example:
Calculate the formula mass of Carbon (i) Oxide.
Carbon (i) Oxide is a compound made of two element; carbon and oxygen.
The atomic mass of carbon is 12 while the atomic mass of oxygen is 16, therefore the formula mass of CO is:
12 + 16 = 28
Therefore, the formula mass of CO is 28 amu.