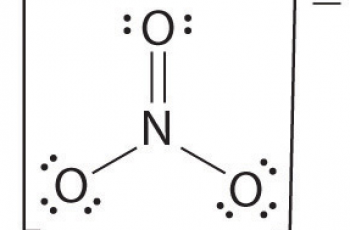
Ka is the acid dissociation constant while Kpa is simply the negative logarithm of Ka.
The dissociation constant for a strong acid can be as high as 10^7 while for a weak acid it can be as low as 10^-12.
pKa is expressed as a common logarithm (base 10) and not as a natural logarithm (base e).
Formula to calculate Ka from pKa.
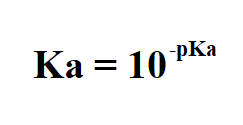
Example:
Suppose the pKa is 7. Calculate the Ka.
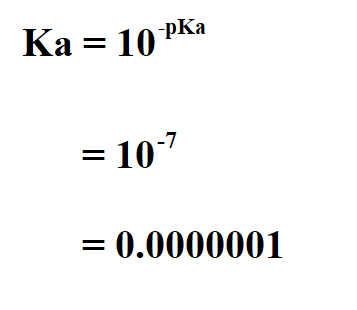
Therefore, the Ka is 10^ -7. This means that this is a strong acid.