Molar absorptivity, also known as the molar extinction coefficient, measures how well a chemical species absorbs a given wavelength of light.
The higher the molar absorptivity, the lower the concentration of species that still gives a measurable absorbance value.
Formula to calculate molar absorptivity.
Using the Beer-Lambert Law we say that;

Example:
Calculate the molar absorptivity of a 2M sodium nitrate whose absorbance rate is 120 and the light path is 0.2 cm.
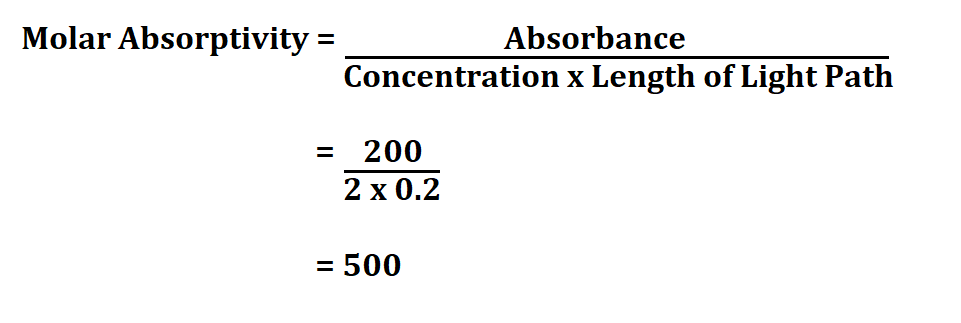
Therefore, the molar absorptivity is 500 L mol^1cm^1.