What is Percent Recovery?
So as to calculate percent recovery, it is essential for us to know its meaning and where this concept is used. Sometimes a Chemist in the lab may perform a procedure where some chemical product can be lost. So he does a purification procedure to recover the lost product. During these procedures, some of the required product may be lost and this results in a reduced recovery.
With that in mind, we can simply define percent recovery as the the amount of pure compound with respect to the impure compound obtained after a purification process. It is normally expressed as a percentage value.
The percent recovery concept is often used in organic chemistry when dealing with the recrystallization processes. This is a process where a chemical is dissolved in a hot solvent and then precipitated out again by cooling the solution, leaving impurities behind.
Additionally, based on Vogel’s Textbook of Practical Organic Chemistry, yields near to 100% are known as quantitative, yields above 90% are known as excellent, yields above 80% are extremely good, yields above 70% are great, yields above 50% are fair, and yields below 40% are known as poor.
Uses of Percent Recovery.
- Percent recovery is used in cases where no chemical reaction is taking place, as in purification of a sample
- We use percent recovery to determine the efficiency of a purification process.
- Percent recovery is very important for industries trying to make the most product with the least waste.
Most people tend to mix up percent recover and percent yield. While percent recovery is calculated as the ratio between the pure compound and initial compound, percent yield is calculated as a ratio between actual yield and theoretical yield.
Formula to Calculate Percent Recovery.
- Weigh the original amount of the substance.
- Complete the purification process.
- On purifying the desired material, leave it aside to dry.
- Remember to remove any other material.
- Weigh the dried substance and record the value.
- Compute the value of percent recovery using the formula below.
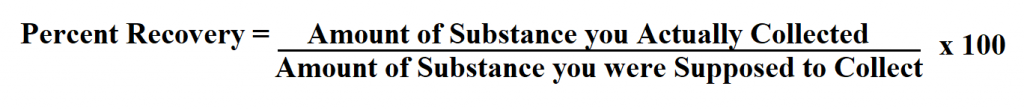
Example 1:
Suppose you had 15g of blue Copper(II) sulfate, after heating it, you were left with 12.8g of white Copper(II) sulfate, Calculate the percent recovery of the compound.

Thus, the percent recovery of the substance is 85.3%.
Example 2:
If 20.0 g of zinc was used in a recrystallization process and the amount of zinc recovered at the end of the process is 18.5 g, calculate the percent recovery.

Thus, the percent recovery is 92.5%.

