In this article we will learn how to calculate relative abundance but before that, let us define an isotope and an atom. Isotopes are different atoms of the same element that contain the same number of protons and electrons but a different number of neutrons.
Simply, isotopes are atoms of the same elements but with different mass numbers.
Atoms are tiny particles consisting of three types of subatomic particles namely electron, proton and neutron. Proton is a positively charged particle, neutrons are not charged and an electron is a negatively charged particle.
Relative abundance is the percentage of a particular isotope with a specific atomic mass that occurs in nature.
The relative abundance of each isotope can be determined using mass spectrometry. A mass spectrometer ionizes atoms and molecules with a high-energy electron beam and then deflects the ions through a magnetic field based on their mass-to-charge ratios
Formula to Calculate Relative Abundance.
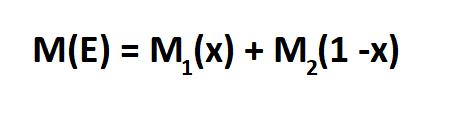
- M1 is the mass of one isotope
- x is the relative abundance
- M2 is the mass of the second isotope
- M(E) is the atomic mass of the element from the periodic table
Example:
Suppose you have a chlorine 35 and 37, each of their masses is 35 and 37, find their relative abundance if chlorine atomic mass is 35.45.
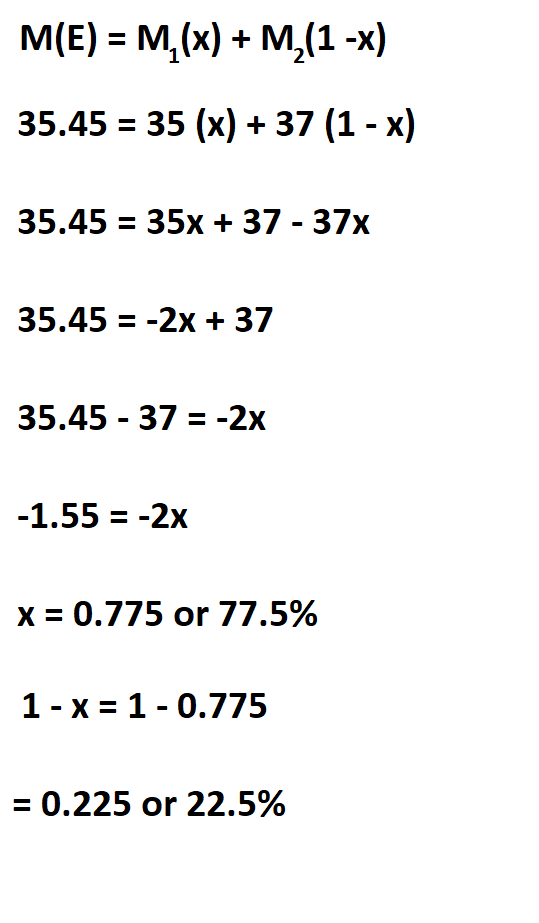
Therefore, the relative abundance of chlorine 35 and 37 is 77.5% and 22.5 % respectively.

