What is Valence Electrons?
Prior to discussing how to calculate valence electrons, let’s us define what it is. Basically, a valence electron (VE) is an electron located in the outermost shell of the atom, this electron(s) can participate in the formation of a chemical bond.
We can obtain the VE from the periodic table because it is equal to the group number of the atom.
The number of valence electrons is important for determining the number of bonds an atom will form, the number of unpaired electrons, the chemical properties of an element and an atom’s formal charge.
An atom with a closed shell of valence electrons tends to be chemically inert while an atom with one or two valence electrons more than a closed shell is highly reactive, because the extra valence electrons are easily removed to form a positive ion.
Additionally, an atom with one or two valence electrons fewer than a closed shell is also highly reactive, because of a tendency either to gain the missing valence electrons or to share valence electrons.
Like an electron in an inner shell, a valence electron has the ability to absorb or release energy in the form of a photon.
Characteristics of VE.
- For the main group elements, the valence electron exists only in the outermost electron shell.
- A valence electron can exist in the inner shell of a transition metal.
- An atom consisting of a closed shell of valence electrons will usually be chemically inert.
- A valence electron can either absorb or release energy in the form of a photon.
- Valence electrons also determine the electrical conductivity of an element. Depending on the nature of elements can be metal, non-metal, or metalloid.
Formula to Calculate VE for Neutral Atoms.
For neutral atoms, the number of valence electrons is equal to the atom’s main group number.
Number of VE = Main Group Number
You can find an element’s main group number from its column on the periodic table.
Example 1:
Carbon is a neutral atom and is found in group 4 of the periodic table, we have seen that number of VE is equal to the main group number, therefore carbon has 4 valence electrons.
Another example is oxygen, it is a neutral atom and it is found in group 6 of the periodic table, this insinuates that oxygen has 6 valence electrons.
Formula to Calculate Valence Electrons for Charged Atoms.
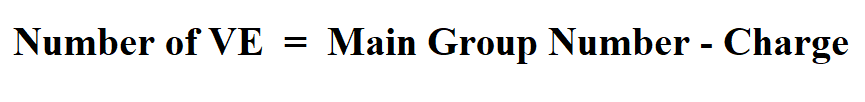
Example 2:
Al+3 normally has 3 valence electrons but with a 3+ charge it is missing three electrons. I n the periodic table , aluminum is a group 3 element.
Therefore,
= 3 – 3
= 0
Hence, Al+3 has 0 valence electrons.
these details dailynewyorktimes.com
I love reading your site.