Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.
Since atoms do not exist in isolation and instead form molecular compounds with other atoms, electronegativity is important because it determines the nature of bonds between atoms.
The most electronegative element is fluorine and has an electronegativity value of 3.98.
Formula to calculate electronegativity.
The Muliken electronegativity equation is as follows;
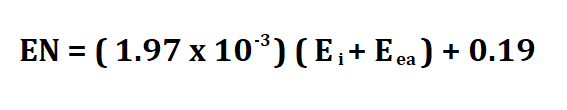
Ei is the first ionization energy (kJ/mol).
Eea is the electron affinity (kJ mol^-1).
Example:
If the first ionization energy of Lithium is 520kj/mol, calculate its electronegativity if its electron affinity is 60 kj mol^-1.
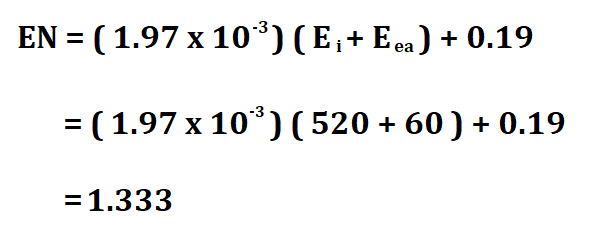
Therefore, Li’s electronegativity is 1.333.