Neutrons are the particles in an atom that have a neutral charge. They are the largest of the particles that make up the atom. The number of neutrons in an atom determines its isotope.
Formula to calculate neutrons.
We know that mass number is the sum of protons and neutrons in the nucleus. The atomic number is the number of protons. Therefore, we can subtract the atomic number from the mass number to find the number of neutrons.
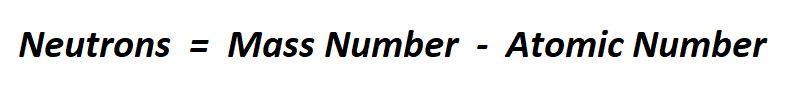
We can get the atomic number and the mass number from the periodic table.
Example:
Let’s consider Sodium (Na) whose atomic number is 11 and atomic mass is 23.
Above, we said that the atomic number is equal to the number of protons.
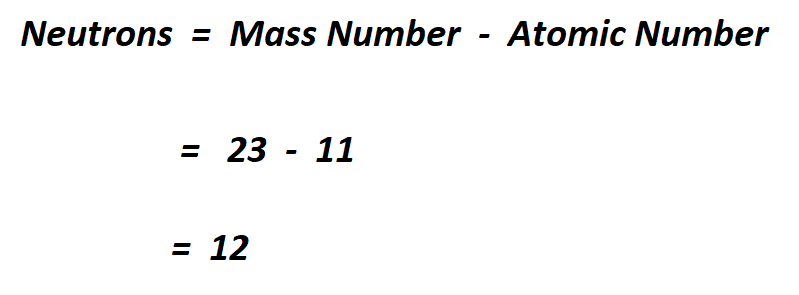
Therefore, the number of neutrons in sodium is 12.
To calculate the number of neutrons in a compound, we first establish what elements make up the compound, then proceed as we have seen above.