The concentration of osmotic-ally active particles in solution, which may be quantitatively expressed in osmoles of solute per liter of solution.
Water moves across a membrane from a lower osmolarity to a higher osmolarity.
Formula to calculate osmolarity.
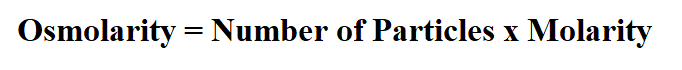
Example:
Suppose you dissolved a 2 molar magnesium chloride compound in water.
The chemical formula for magnesium chloride is MgCl₂, therefore it means, when you dissolve it in water it dissociates into 2 particles of magnesium and one particle of chloride. Therefore, it has 3 particles.
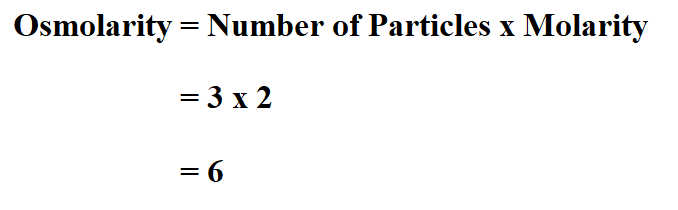
Therefore, the osmolarity is 6 osmol.