The pKa value is one method used to indicate the strength of an acid.
A lower pKa value indicates a stronger acid. That is, the lower value indicates the acid more fully dissociates in water.
Formula to calculate pKa.
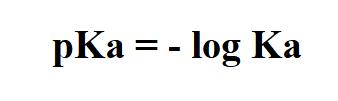
Example:
Calculate the pKa of hydrochloric acid if its Ka is 10^7.
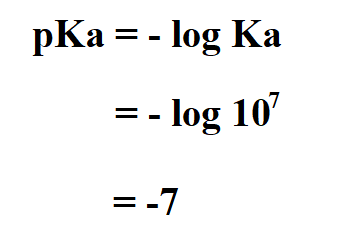
Therefore, the pKa of HCl is -7.

